Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biologics
Covid-19
Convalescent plasma data trickle out from COVID-19 studies, but scientists wonder if results will come too late
Scientists face logistical and technical hurdles in trying to prove that the experimental treatment saves lives
by Ryan Cross
June 11, 2020
| A version of this story appeared in
Volume 98, Issue 23

In April, Elliott Bennett-Guerrero began one of the country’s most advanced studies of one of the oldest weapons in the antiviral armamentarium. The anesthesiologist and intensive care unit doctor at Stony Brook Medicine in New York had been working with his hospital’s blood bank to start collecting convalescent plasma, the antibody-rich fraction of blood obtained from people who had recovered from COVID-19, the disease caused by the novel coronavirus SARS-CoV-2.
The goal was simple: to figure out if giving convalescent plasma to people hospitalized with COVID-19 can keep them alive and off a ventilator. The need was urgent. “I have never seen such sick patients in my 25 years working in an ICU,” Bennett-Guerrero says. “It is really tragic.”
In medical lingo, the word convalescent refers to a person undergoing recovery from an illness, and the crux of convalescent plasma is that if there is something in the blood that’s helped one person get better, it might help someone else get better too. That something is an antibody, or rather an arsenal of antibodies, that target SARS-CoV-2.
After getting the green light from the US Food and Drug Administration, Bennett-Guerrero began his clinical trial on April 17. In the weeks since, the use of convalescent plasma has exploded. There are upward of 80 active or planned studies to use convalescent plasma to treat or prevent COVID-19. Local news reports are peppered with stories of people donating convalescent plasma after recovering from COVID-19, or of people recovering from the disease after receiving a convalescent plasma infusion.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
Doctors have intermittently tried using convalescent plasma to treat infectious diseases for more than 100 years. Yet in all its storied history, from the Spanish Flu pandemic of 1918 to the Ebola outbreaks of the past decade, the attention that the treatment is receiving now is unparalleled, says Michael Busch, director of the Vitalant Research Institute, part of Vitalant, the nation’s second largest blood bank, which is collecting convalescent plasma. “We have never scaled up even close to what’s happening now,” he adds.
Anecdotal evidence from China early in the pandemic suggested that convalescent plasma might help people with COVID-19 recover more quickly. Since the FDA still considers the treatment experimental, Bennett-Guerrero decided to put it through the wringer to try to prove that it works. In his study, up to 400 people will receive convalescent plasma, while 100 others will receive regular plasma as a control. Only the blood bank—and neither the doctor nor the patient—will know which infusion each patient gets.
The Stony Brook study is what’s known as a double-blind, randomized, controlled clinical trial. It’s the gold standard for proving whether a treatment is effective or not.
But such trials are surprisingly rare for convalescent plasma. Many smaller studies are open-label—meaning everyone knows who is getting a treatment—and do not include a group that receives a comparable control. As of early June, the vast majority of the more than 20,000 COVID-19 patients in the US who have received convalescent plasma infusions have received treatment through a national compassionate use program spearheaded by the Mayo Clinic.
Several other factors will make assessing the usefulness of convalescent plasma difficult. Not all convalescent plasma is created equal; some people produce enormous levels of antibodies, while others seem to produce virtually none. And clinics are characterizing the inherent variability of convalescent plasma under a hodgepodge of standards. While some groups use only plasma with high antibody concentrations, many are administering plasma without first testing it for antibodies. Even labs that do measure antibody levels do so with a menagerie of methods.
These issues will complicate the interpretation and comparison of convalescent plasma studies. Yet even if the treatment is effective, it will face competition. This summer, several pharmaceutical companies are launching clinical trials of monoclonal antibodies—standardized drugs that target SARS-CoV-2 and can be manufactured without the reliance on plasma donations.
In the end, convalescent plasma’s biggest barrier may be time. The clock is ticking, and what happens next could not only change the course of this pandemic, but pandemics to come.

Quantitative quandaries
When the coronavirus wreaks havoc in someone’s body, their immune system fights back. As part of that counterattack, a brigade of immune cells make antibodies that target the virus. A small subset of those antibodies, called neutralizing antibodies, directly prevent the virus from infecting any more cells. Most others are binding antibodies, which simply stick to the virus and help flag down additional immune cells that break the virus apart or engulf it whole.
Some people’s immune systems are more successful at this endeavor than others. Convalescent plasma therapy assumes that if someone recovers from an infection, they must have mounted a good immune response, and that transferring this immune response—vis-à-vis antibodies—to a sick person can provide them a boost.
Early in the pandemic, a group of doctors and infectious disease specialists made plans to rapidly collect and distribute convalescent plasma. They viewed plasma as a stopgap measure until novel antivirals and vaccines to treat and prevent the coronavirus became available.
Throughout April and May, more than a dozen reports of doctors using convalescent plasma to treat COVID-19 began to trickle out in peer-reviewed journals and on the preprint server medRxiv. Some described harrowing rescues bringing elderly individuals back from the brink of death. Others reported that nearly everyone on ventilators died despite receiving plasma.
None offered conclusive evidence for or against plasma—partly because most of these reports are anecdotal, and partly because nearly everyone received a medicine cabinet’s worth of treatments including antivirals, antimalarial drugs, and anti-inflammatory drugs in an attempt to keep them alive. In theory, convalescent plasma should work. But despite its 100-plus years of history, the data supporting its efficacy are surprisingly weak.
Authors of the most recent meta-analysis, published in May, found that substandard design was so pervasive in coronavirus and influenza convalescent plasma studies that they couldn’t make confident conclusions about the treatment’s ability to prevent death. “It is all very poor quality evidence,” says Philippe Bégin, a doctor running a convalescent plasma trial at CHU Sainte-Justine in Montreal, and one of the study’s authors.
Convalescent plasma studies are nearly always small and hastily planned. “People pull this out as an option for dire circumstances when you don’t know what else to do, and those aren’t great circumstances to design a controlled study,” says Jeffrey Henderson, an infectious disease biochemist at Washington University in St. Louis.
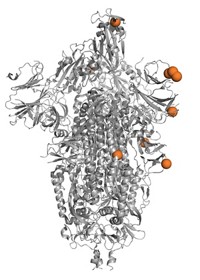
Scientists believe that one of the biggest determining factors for convalescent plasma’s potential success, or failure, is the quantity and quality of antibodies. Most of the antibodies that our immune cells make in response to SARS-CoV-2 target the virus’s spike protein. An array of these large spike proteins protrude from the viral surface and attach to a receptor called ACE2 on human cells. A sliver of our antibodies bind to a particular zone of the large viral protein, called the receptor binding domain (RBD). These anti-RBD antibodies can physically block the spike’s interaction with ACE2.
And a further subset of these anti-RBD antibodies, the so-called neutralizing antibodies, are highly effective at preventing SARS-CoV-2 from infecting human cells grown in a dish. A few studies have even shown that some, but not all, of these antibodies can prevent or halt infection in lab animals exposed to the virus.
“There is a wide variation in neutralizing antibody responses in infected individuals,” says Dennis Burton, an immunologist at Scripps Research. Although his group, and others, have found highly potent neutralizing antibodies in the convalescent plasma of many people, the levels of these antibodies are often very low, he says.
Scientists can measure the levels—also called titers—of antibodies in a convalescent plasma sample by repeatedly diluting the sample and using a standard test called ELISA (enzyme-linked immunosorbent assay) to see if the antibody is still detectable. For instance, a 1:320 titer contains about twice as many antibodies as a 1:160 titer. The FDA recommends convalescent plasma contain a neutralizing antibody titer of at least 1:160, a standard some scientists have criticized as arbitrary. “People assume that because it is the criteria set forth by the FDA it has some scientific basis, but it doesn’t,” Bégin says.
Bégin worries that the threshold could prevent the field from learning whether lower titers could be effective. The bigger problem, however, may simply be that early in the pandemic, most groups did not even measure antibody titers of their plasma. Those that did used a gamut of standards. “People are using tons of different tests,” Bégin says. “And depending on the method you are using, you can get very different readouts.”
Some simply measure anti-spike titers, while others look for the more specific anti-RBD titers. Bennett-Guerrero’s study is measuring antibody levels of a different SARS-CoV-2 protein entirely, called the nucleocapsid. Studies that test for the all-important neutralizing antibody titers, however, are rarer, because they’re considerably more difficult to measure.
Traditionally, scientists quantify neutralizing antibody levels by exposing petri dishes of cells to live viruses, and seeing what dilution of antibodies prevents infection. Such an experiment with live SARS-CoV-2 is dangerous, expensive, and must be done in a high-security lab. As a result, several groups are developing safer, more accessible tests that use an artificial version of the virus and a fluorescent marker to measure neutralizing antibody titers.
There’s also a chance that neutralizing antibodies are not the only important factor for success. The vast majority of antibodies that target SARS-CoV-2 simply bind to the virus—they don’t neutralize it. Some even seem to bind to the virus after it infects our cells. These various binding antibodies may help slow an infection through several indirect mechanisms, such as recruiting the immune system to destroy the infected cells or the virus itself.
Early signals
In early June, a large group of scientists from China published the world’s first data from a randomized clinical trial of convalescent plasma therapy. The study compared the outcomes of 103 hospitalized COVID-19 patients receiving convalescent plasma to those who just got standard care.
The study reported that 52% of patients who received plasma improved within 4 weeks, compared to 43% of patients in the control group, although the results were deemed statistically insignificant. The study was supposed to include 200 people, but it got cut short as the pandemic waned in China in late March. Even though the study was no sweeping success, Bennett-Guerrero says “it is promising that the results are in the right direction.”
Preliminary results are also emerging from convalescent plasma studies conducted in the US.
The Mayo Clinic, which with the American Red Cross and the FDA established a national program to provide convalescent plasma to doctors around the country, recently offered a preliminary report of the first 5,000 transfusions. Although the program does not include a control arm, their early data suggest the treatment is largely safe, although not without risk. Transfusions can cause rare pulmonary injuries or allergic reactions. 21 people had serious reactions to the plasma, and 15 people died within 4 hours of transfusion—although the scientists could not confidently link those deaths to the plasma. The study has not been peer reviewed.
Advertisement
In a 39-patient study at the Icahn School of Medicine at Mount Sinai published recently on the preprint server medRxiv, convalescent plasma improved survival in some hospitalized patients, but not ones requiring mechanical ventilators. Only 12.8% of people receiving convalescent plasma died in the study, compared to 24.4% of a control group of similar patients whose data was obtained from the hospital’s electronic health records.
And a study from Houston Methodist Hospital found that convalescent plasma may have improved the health of 19 out of 25 people with severe or life-threatening COVID-19 after 2 weeks, although everybody in the study also got hydroxychloroquine and azithromycin treatments, and more than half received other drugs as well.
Bennett-Guerrero originally hoped to finish his study at Stony Brook by early summer. Its size, 500 patients, and robust design could make it one of the first trials that has a shot at proving convalescent plasma works. But so far, only 70 patients have enrolled. “COVID-19 admissions have come down to a trickle,” he says. “We didn’t just flatten the curve in New York, we squashed it.”
That good news will make it harder for doctors like Bennett-Guerrero to complete their convalescent plasma studies. It could also mean that fewer people will receive the plasma through Mayo’s expanded access umbrella. The FDA only allows doctors to give convalescent plasma to patients through the expanded access pathway if they have severe or immediately life-threatening COVID-19. “It may be too late for these patients,” Henderson says. If the viruses’ damage is already done, and an individual’s own runaway immune system is doing the damage, antibodies may not make a difference.
Doctors have to participate in a clinical trial to give convalescent plasma to less severely ill patients. Washington University in St. Louis is conducting several such trials, Henderson explains, including one designed to keep people off of ventilators, and a prophylactic trial to give the plasma to people that may have been exposed to the virus, such as health-care workers, to see if the plasma can prevent, or at least blunt, an infection.
Large trials in other countries will still seek evidence for whether convalescent plasma helps hospitalized patients survive and stay off ventilators. CONCOR-1, a randomized controlled trial co-led by Bégin, will compare convalescent plasma treatment in 800 people to standard treatment in 400 people in about 50 sites across Canada and the US. And two large trials across the UK—the RECOVERY trial and the REMAP-CAP trial—will collectively test convalescent plasma in thousands of hospitalized and intensive care patients, respectively, and compare its effectiveness to other experimental treatments such as antivirals, anti-inflammatory drugs, and hydroxychloroquine.
All of these trials will be competing for patients that could soon opt to enroll in studies of hyperimmune plasma—convalescent plasma that’s been pooled from multiple patients and divvied up into doses with consistent antibody titers—or of monoclonal antibodies specifically designed to neutralize the virus. Thomas Gniadek, an assistant director of transfusion medicine at NorthShore University HealthSystem in Illinois, worries that convalescent plasma will become irrelevant before scientists have solid data on whether it works or not. “There was a window, and I think the window will be closing very quickly,” he says.
Scientists stress the importance of knowing whether convalescent plasma is effective. If all the newly designed therapies and vaccines fail, it could still be a useful tool for doctors. And running these experiments will determine whether we can rely on convalescent plasma in the event of another wave of SARS-CoV-2, or another coronavirus pandemic.
“This is not a treatment that is designed to be used indefinitely. It is meant to be used until we have a vaccine, small molecule, or monoclonal antibody,” Henderson says. “I see it as a series of hand-offs. But I think there’s potential that convalescent plasma gets us out of hiding in our homes.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter